Around July 12, several media outlets from all over the world reported that Russia’s Sechenov University has completed clinical trials of COVID-19 vaccine. These reports also suggested that this is the first vaccine in the world to complete human trials. A list of media outlets that published such reports can be accessed here. The Twitter account of the Russian Embassy in India had shared one such report.
🦠#Sechenov University has successfully completed tests on volunteers of the world’s first vaccine against #COVID19.
“The #vaccine is safe. The volunteers will be discharged on July 15 and July 20″, chief researcher Elena Smolyarchuk told TASS ➡️ https://t.co/jVrmWbLvwX pic.twitter.com/V8bon4lieR
— Russia in India (@RusEmbIndia) July 12, 2020
Indian media outlets
News agencies ANI and IANS published reports claiming that Sechenov University has completed clinical trials of COVID-19 vaccine. This was republished by several other media outlets. Other leading news organisations that published the viral claim were — Times of India, The Print, Financial Express and India Today.
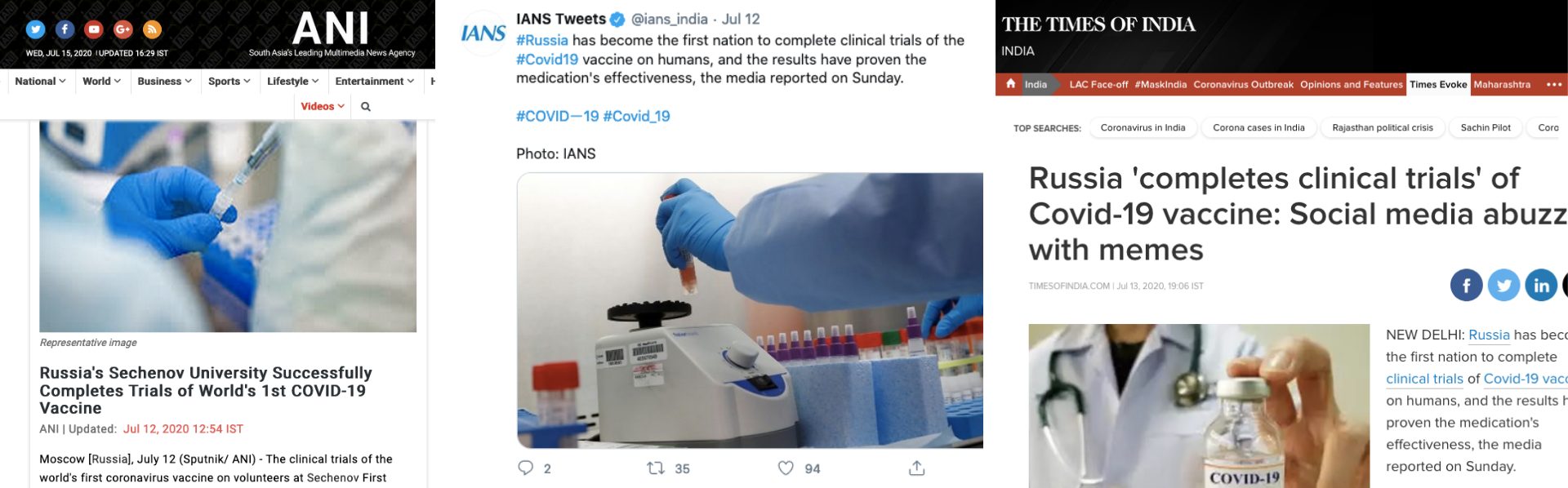
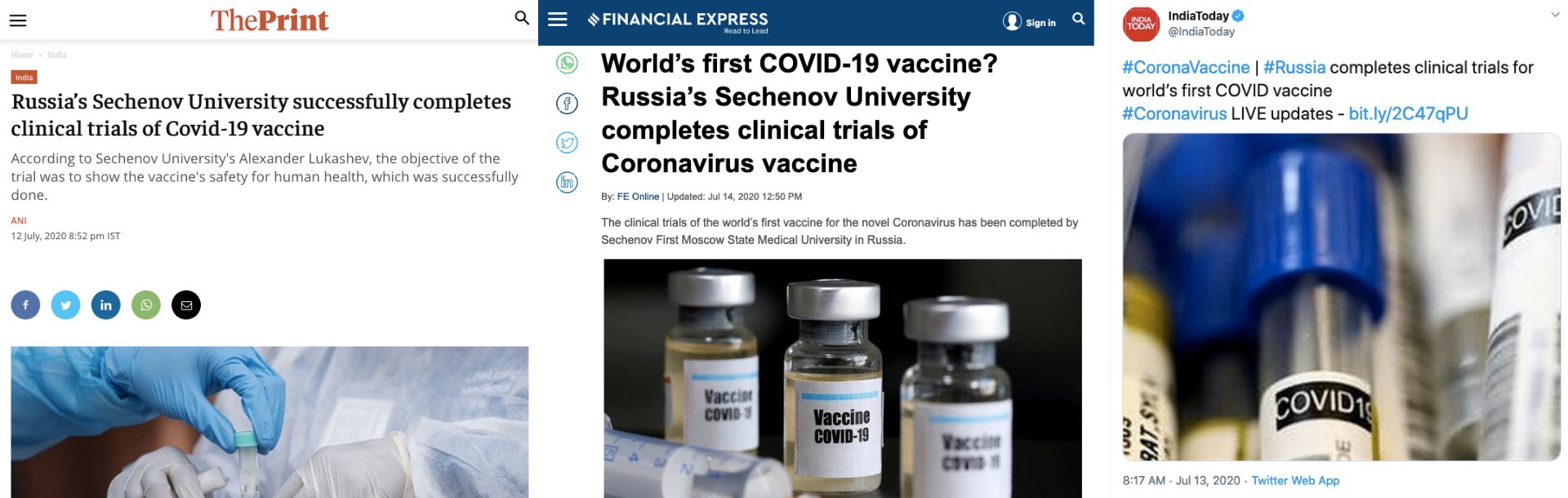
News 18, Bangalore Mirror, Hindustan Times, WION Outlook, Livemint, International Business Times, ET Now, National Herald, India TV, Business Standard, Business Insider India, Economic Times, Editorji, Yahoo India, LatestLY, The Logical Indian and Swarajya were the other outlets that carried similar reports.
International media outlets
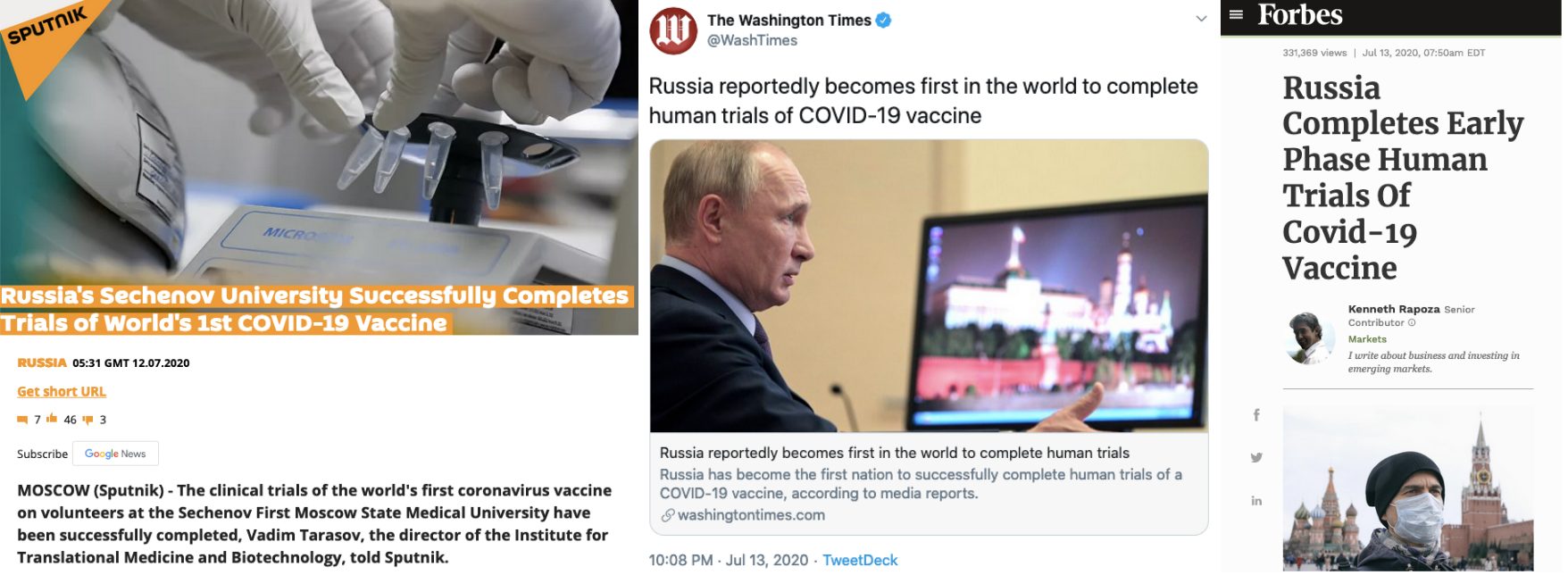
Several international media organisations also claimed the same — Sputnik, The Washington Times, Forbes, Newsweek and PM News Nigeria. It is noteworthy that ANI credited its report to Sputnik.
Journalists
National Bureau chief of ANI Naveen Kapoor had tweeted the claim on July 12.
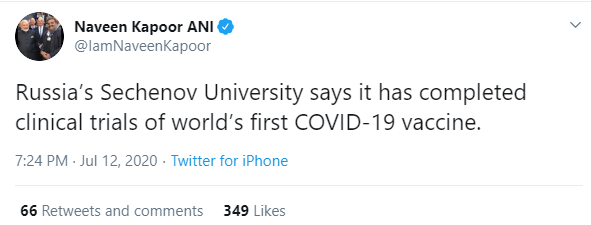
So did several other journalists including editor at Ohio-based Richland Source Larry Phillips, author at Swarajya and The Times of India Aashish Chandorkar and film correspondent at Sify and author at Firstpost Rajashekar.

Claim
1. Sechenov University has completed clinical trails of COVID-19 vaccine.
2. Russia is the first country to complete COVID-19 vaccine human trials.
Fact-check
1. Sechenov University has completed the first phase of clinical trials of COVID-19 vaccine
As per the World Health Organization, clinical trials are a type of research that study new tests and treatments and evaluate their effects on human health outcomes. There are 4 phases of biomedical clinical trials:
Phase I studies usually test new drugs for the first time in a small group of people to evaluate a safe dosage range and identify side effects.
Phase II studies test treatments that have been found to be safe in Phase I but now need a larger group of human subjects to monitor for any adverse effects.
Phase III studies are conducted on larger populations and in different regions and countries and are often the step right before a new treatment is approved.
Phase IV studies take place after country approval and there is a need for further testing in a wide population over a longer timeframe.
On July 12, Telegraph Agency of the Soviet Union (TASS) reported, “The first stage of research on the vaccine at Sechenov University kicked off on June 18 when a group of 18 volunteers were vaccinated. The second group of 20 volunteers were vaccinated on June 23.” However, it’s pertinent to note that the first sentence of the report could have contributed to widespread misnformation. It said, “Clinical trials of vaccine against the novel coronavirus were completed on volunteers at Sechenov University.”

According to Firstpost, “The vaccine is being developed and produced by the Gamaleya Scientific Research Institute of Epidemiology and Microbiology along with the Russian defence ministry. The trials are being conducted in the Sechenov University.”

The COVID-19 vaccine tracker created by the WHO states that the vaccine is in Phase 1/2 of clinical trials. “In the Russian vaccine trials, the first stage of research on the vaccine began on 18 June when a group of 18 volunteers were vaccinated. The second stage had 20 volunteers and they were vaccinated on 23 June,” reports Firstpost. The website ClinicalTrials.gov, which is a US registry of all the ongoing clinical studies, states that Phase I trials of the vaccine have been split into two stages. “The trials are split into two half as the two groups are being given different doses of the vaccine,” Firstpost further states.
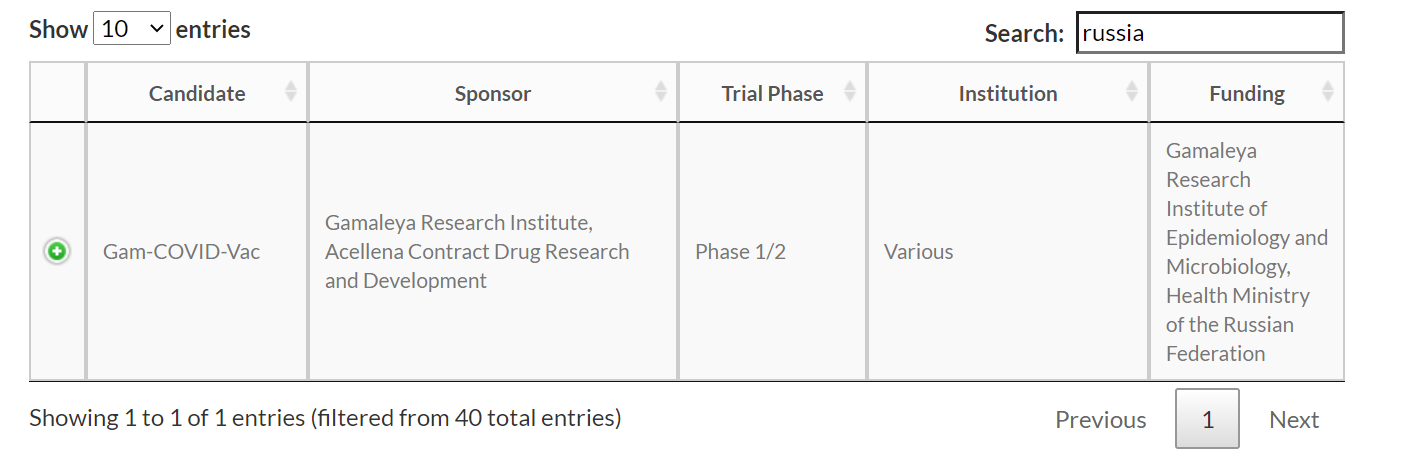
Thus Sechenov University has completed the first phase of clinical trials of COVID-19 vaccine.
2. Russia is not the first country to conduct human trials
On March 16, Associated Press had reported that the first human trials were conducted at the Washington Health Research Institute in Seattle, US. According to WHO COVID-19 vaccine tracker, the institute has progressed to Phase 2 of clinical trials.
Thus, the claim that Russia is the first country in the world to “complete” human trials is also false.
Conclusion:
Media outlets across the world carried misreports that Russia’s Sechenov University has completed clinical trials of COVID-19 vaccine. The trials are in Phase I. Furthermore, it is also false that Russia is the first country in the world to conduct human trials. Washington Health Research Institute in Seattle, US had reportedly conducted the first human trials in March.
Independent journalism that speaks truth to power and is free of corporate and political control is possible only when people start contributing towards the same. Please consider donating towards this endeavour to fight fake news and misinformation.