Patanjali Ayurved launched an ayurvedic treatment kit called ‘Coronil’ as a ‘cure’ for COVID-19. Indian media outlets ran shows on the kit, referring to it as a ‘breakthrough’ in coronavirus treatment. Ram Kisan Yadav, popularly known as Baba Ramdev, the face of Patanjali, stated that the kit was developed using “clinically controlled trial” and claimed that it was a “100% cure for COVID-19”. Further, Ramdev stated that they had all the required permissions for manufacturing and releasing the drug.
Subsequently, the AYUSH ministry issued a statement that Patanjali Ayurved has been asked not to advertise the drug as a cure for COVID-19 due to the lack of documents (i.e. proof of efficacy). Ramdev claimed in an interview with India Today that by evening, AYUSH will be sent all the documents related to the research that has been used as the basis of the proof of efficacy for Coronil kit.
Patanjali released a set of documents that included:
- The previously circulating clinical trial registry document (CTRI), which was registered on May 20, 2020.
- A letter to the AYUSH drug policy section that detailed the composition of the drug, the details of the clinical trial and where it was conducted.
- Two links of the research articles enlisted within the composition of the drug.
- And letters of the ethics approval committee, and two FYI letters to the Director-General, CCRAS and Additional Chief Secretary, Ministry of Health & Family Welfare.

Thus, apart from the CTRI, only the two research articles were listed as proof of efficacy that the drug provides 100% cure from COVID-19. Further, the license officer of Ayurved Department in Uttarakhand has stated that Patanjali’s application was approved for use as an immunity booster and not a drug for COVID-19.
In this sci-check, we will look at the composition of Coronil kit and the two studies listed in the Patanjali document.
Claim:
The Coronil kit by Patanjali Ayurved is a 100% cure of COVID-19 and has been scientifically tested with clinical trials.
Verdict:
False
Fact-Check:
The Coronil Kit contains three bottles of:
- Swasari Ras tablets or (DRS) — a herb mixture tablet.
- The herb mixture is two-part Giloy, one part Ashwagandha and one part Tulsi.
- Anu Taila (nasal oil drops).
Apart from the CTRI which is a registration of the Clinical Trial (not a published RCT study), only the two research studies were listed in the document given to AYUSH as proof of efficacy of the drug. None of these studies was the published randomised controlled trials (RCTs) as stated by Patanjali.
Among the two studies, the first was related to tablet 1 (DRS) in the kit, and the second study was related to only one of the herbs in the Coronil tablet called Giloy. There were no studies in the document provided for Ashwagandha and Tulsi, the other two components of the Coronil tablet. Also, no study was provided for the third drug in the kit Anu Taila (nasal oil drops).
Further, the CTRI document suggested that 120 COVID-19 infected patients received Coronil treatment and Patanjali has claimed that they were successfully cured at the rate of 100%. However, the CTRI document is only proof of registration of the clinical trial and not proof of results found within the trial. Proof of efficacy should be a published, peer-reviewed study by the research standards in a sizable population.
In addition, the following two studies were referenced as the scientific evidence by Patanjali to prove the efficacy of Swasari Ras (1st drug of the kit) and Giloy (a part of Coronil tablet or 2nd drug of the kit) for COVID-19.
This study evaluated the effect of Swasari Ras or Divya Swasari Ras (DSR), in mice (not humans) induced in a lab in an allergic asthma model. The drug DSR has neither been tested on inflammatory lung tissue with coronavirus nor was it tested on humans. Allergic Asthma, on which DSR was tested on as part of this study and the disease due to the novel Coronavirus (COVID-19) are completely different diseases, and the mechanisms of the diseases are different as well. Moreover, since the tests for DSR are conducted in non-human species, the study cannot be used as a 100% proof to claim the efficacy of DSR against COVID-19.
The second study is a preprint (i.e. a non-peer-reviewed article), which hasn’t been reviewed by other scientists to ascertain its scientific validity. During this pandemic, there are many pre-prints available due to the speed of the information necessary to hasten the research process. This particular study released by Patanjali Ayurved as a 100% proof of efficacy for Coronil tablet is a molecular study of Giloy herb (Tinospora Cordifolia) through a computer simulation and is not a study on any living species, mice or humans.
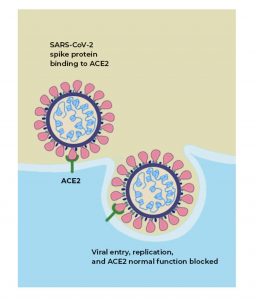
Since the Coronavirus (SARS-COV2) binds to the host cell in humans via its ACE receptor using a spike protein, the study claims that Giloy disrupts this binding by interrupting its electrostatic interactions between the ACE receptor and the receptor-binding protein (RBD) (see image). Both ACE receptors and spike protein RBD are the proteins used by SARS-COV2 to gain entry into the cell.
The study is simply a molecular dynamics (MD) ‘simulation’ which is a computer simulation, and in this case, was conducted using software called Discovery Studio, Biovia.
While the study needs peer-review, it is neither a ‘clinical trial’ as claimed by Ramdev or Patanjali, nor does it explore the interactions between the two proteins in humans or any other living species
Conclusion:
Therefore, none of the scientific proofs given by Patanjali suggests that Coronil is an effective treatment for COVID-19 for the following reasons:
- The documents provided have published research study for only one out of the three drugs in the Coronil kit, and a non-peer-reviewed study for only a part of the herbal mix (Giloy) of three-herb Coronil drug.
- As per the manufacturing or approval of evidence-based medicine, a non-peer review study for only a part of the drug cannot be taken as proof of efficacy for the Coronil tablet.
- The first research study for Swasari Ras (DSR) is a study on mice and not humans.
- Also, the second research study for Giloy is a computer simulation and not a study on any living species.
- The clinical trial registry document (CTRI) provided is only a proof of registration and not proof of results found within the trial.
- Further, no toxicity or herbal interaction study was conducted to prove that the drug combination was safe or effective in COVID-19 patients.
In fact, claiming that such untested drugs will cure a 100% of COVID-19 patients amounts to a false assumption of treatment and depletes them of the opportunity to receive proven treatment where necessary, even though they may not always treat every patient. This practice of false drug manufacturing under the garb of ancient ayurveda endangers the lives of people during the times of a global crisis.
References:
Balkrishna, A., Solleti, S. K., Singh, H., Tomer, M., Sharma, N., & Varshney, A. (2020). Calcio-herbal Formulation, Divya-Swasari-Ras, Alleviates Chronic Inflammation and Suppresses Airway Remodelling in Mouse Model of Allergic Asthma by Modulating Pro-Inflammatory Cytokine Response. Biomedicine & Pharmacotherapy. 2020 Jun;126:110063.doi: 10.1016/j.biopha.2020.110063
Acharya Balkrishna, SUBARNA POKHREL, Anurag Varshney. Tinospora cordifolia (Giloy) may curb COVID-19 contagion: Tinocordiside disrupts the electrostatic interactions between ACE2 and RBD. Authorea. April 16, 2020. DOI: 10.22541/au.158707095.53639175
Independent journalism that speaks truth to power and is free of corporate and political control is possible only when people start contributing towards the same. Please consider donating towards this endeavour to fight fake news and misinformation.