On October 31, the Food Safety and Standards Authority of India (FSSAI) upheld its earlier ban on beverages misleadingly labelled as ORS (oral rehydration solution), calling it a public “health hazard”. This essentially means that any beverages — fruit-based, non-carbonated or otherwise — that do not follow the World Health Organisation (WHO)-recommended proportions for the electrolyte-based medical formulation cannot use the term ‘ORS’ in their products or branding.
The FSSAI first issued the directive on October 14, but this was challenged in the Delhi High Court by pharma majors who claimed the sudden order left them blindsided. In the October 31 hearing, the Delhi High Court, which was hearing a plea by pharma major Dr Reddy’s Lab challenging the FSSAI order in a bid to exhaust its existing stock of ORS-labelled products, refrained from interfering with the statutory body’s order. Besides Dr Reddy’s, JNTL Consumer Health (India) Private Limited (a Johnson & Johnson subsidiary), which manufactures the product ORSL, had also challenged FSSAI’s ban.
The latest directive follows a long fight by several doctors and medical professionals, particularly a paediatrician named Dr Sivaranjani Santosh, who has, since 2017, been documenting cases of sugary drinks being packaged as ORS and how that has led to detrimental effects on children suffering from diarrhoea. Dr Sivaranjani filed a Public Interest Litigation (PIL) in 2022 in the Telangana High Court against companies labelling beverages as ORS and highlighted in her petition that these products contained up to 120 grams of sugar per litre — far higher than the 13.5 grams per litre recommended by the WHO.
Let us look at the latest set of hearings and why mislabelling of the product is such a major health hazard.
A Timeline of the Case
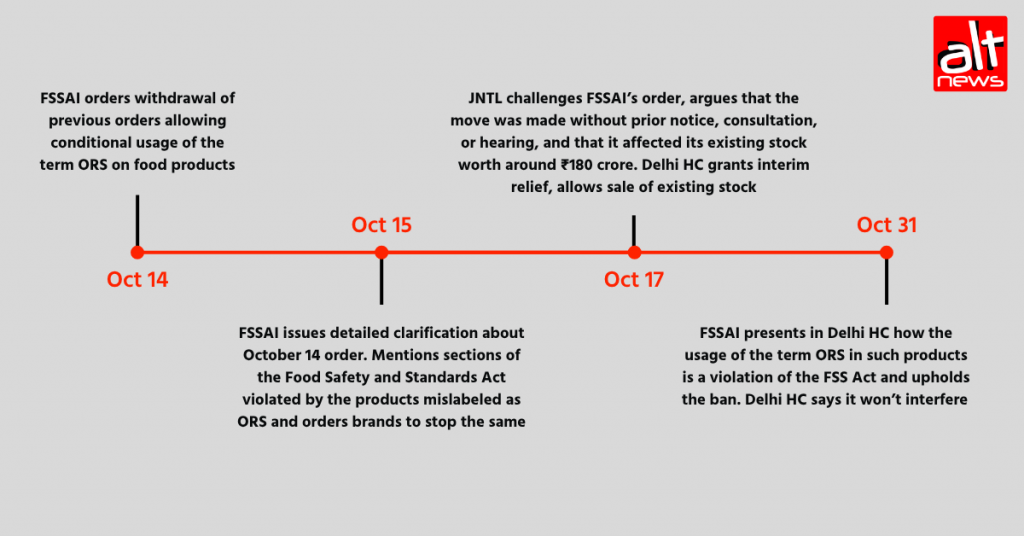
On October 14, the FSSAI issued an order, withdrawing its older directives dated July 14, 2022, and February 2, 2024, in which the body had allowed the conditional use of the term ‘ORS’ on food products, as long as the package carried a clarification that the product did not adhere to WHO’s formulation.
On October 15, the body released a detailed clarification stating which sections of the Food Safety and Standards (FSS) Act, 2006, these products violate and directed that “Food Business Operators remove the word ‘ORS’ from their food products, whether used as a standalone term or in combination with any prefix/suffix or as part of the trademark with prefix/suffix in the product name”. However, it did not explicitly mention whether existing stock was to be taken off the shelves or the directive adhered to newer production batches.
Then, on October 17, JNTL challenged the above-mentioned FSSAI order and filed a petition in the Delhi High Court stating that the order was abrupt and was rolled out without any prior notice or consultation. The J&J subsidiary, which sells the product labelled ‘ORSL’, claimed that it affected its existing stock worth around Rs 180 crore.
Following this, the Delhi High Court granted interim relief to the company and gave the FSSAI a week’s time to present its case. Until then, the October 14 and 15 FSSAI orders would not be implemented on JNTL products — FSSAI agreed to this, too.
This invited much criticism from health experts who felt that the long-overdue ban hit another roadblock and would not be implemented amid pharma corporations’ lobbying efforts.
In an X post on October 23, Dr Cyriac Abby Philips, also known as ‘The Liver Doc’ (@theliverdr), said: “…The Court and FSSAI has essentially allowed the sale of fake mislabelled products worth 180 crore Indian Rupees to protect the interest of the greedy corporations without any concern for the children’s health…”
Dr. @dr_sivaranjani painstakingly fought for 8 years to convince the Food Safety and Standards Authority of India (FSSAI) to heed the harms of fake ORS drinks targeting children, flooding the market from greedy corporate companies.
She won. FSSAI ordered the ban on the use of… pic.twitter.com/Ln5cwwR2Bc
— TheLiverDoc™ (@theliverdr) October 23, 2025
Dr Sivaranjini Santosh, who has been at the forefront of the case, also posted a video on October 18 saying, “These people should not be allowed to sell their 180 crores stock at the cost of lives of children!”
These people should not be allowed to sell their 180 crores stock at the cost of lives of children! Tag @indSupremeCourt @fssaiindia @MoHFW_INDIA @OfficeofJPNadda @narendramodi
— Dr.Sivaranjini (@dr_sivaranjani) October 19, 2025
Amid the backlash, Justice Sachin Datta, who heard the case, clarified that the order did not intend to allow companies to continue producing products under the label ‘ORS’ to mislead, but the stay was for the FSSAI to step in as the statutory body dealing with food and beverages:
“I would have restrained the manufacturers from manufacturing fresh batches had I known that the FSSAI would take two weeks to make a decision. The consent order was passed to enable FSSAI to take the requisite steps. It wasn’t to allow these manufacturers to continue manufacturing the products. The idea was that the FSSAI would do the needful in two to three days,” he said.
Finally, on October 31, after FSSAI’s representation at the Delhi High Court, the ban on products mislabelled as ‘ORS’ was upheld.
How Mislabelling Sugar-Laden Drinks as ORS Can Be Lethal
Apart from the obvious demerits of consuming high-sugar beverages, why is consuming ORS that does not adhere to the WHO’s formulation lethal in some cases?
ORS, or Oral Rehydration Salts, also known as Oral Rehydration Therapy (ORT), is a fluid replacement solution containing a balanced mix of sugars, salts, sodium, and potassium. It is used to prevent and treat dehydration caused by illnesses such as diarrhoea. ORS intervention has been shown to decrease the risk of death due to diarrhoea.
However, according to FSSAI’s updated order, “The ingestion of a high-sugar electrolyte drink can worsen dehydration rather than alleviate it, by drawing water out of body cells through osmotic imbalance. The risk is further aggravated among children, diabetic patients, and elderly persons, who represent the most vulnerable categories of consumers. Accordingly, the said product poses a direct and immediate risk to human health as it induces consumers to believe that the product is intended for medical or rehydration use.”
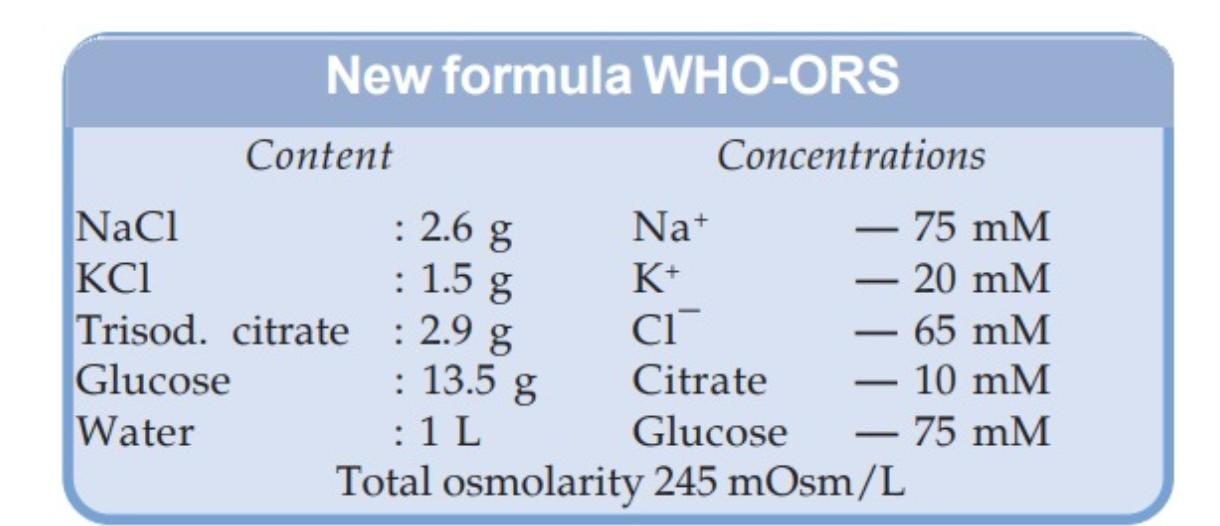
Below is the WHO-recommended composition used for intervention in case of dehydration in a patient. According to this, the total osmolarity of the electrolyte drink should be 245 mOsm/L and for that, the sodium and glucose levels need to be maintained as well as the water level, which was not being maintained by several non-WHO products labelled ‘ORS’.
To understand more, we also reached out to Dr Abhishek Chowdhury, a consultant pathologist with a sub-specialisation in neuropathology, who talked to us about the damaging effects of ORS mislabelling.
“The 75mM proportions mentioned for sodium and glucose have to be mixed with 1 litre of water. If one mixes these proportions in, say, 500 ml or 200 ml of water, then the correct ratio required for oral rehydration solution isn’t being maintained, and hence, it won’t do the job it is required to do. The problem is, these brands in the market, which are selling 200 ml pouches (disguised as ORS), have extremely high sugar content. Even the original WHO formula previously had a higher osmolarity; it was around 300, but WHO brought it down to 245, because it had been observed in studies that lower osmolarity showed better electrolyte absorption. The presence of high sugar pulls water into the intestine and increases dehydration, as it is the inherent property of glucose, to draw water and sodium along with it”, Dr Chowdhury told Alt News.
Dr Chowdhury also explained that the ingestion of these products (labelled like ORS but not adhering to the WHO formula) could cause dehydration and loss of fluid, which is far more difficult to gauge in children, as they might not be able to voice what they are feeling, and could lead to serious conditions very quickly. For diabetic patients suffering from dehydration, it can also lead to severe hyperglycemia and even diabetic ketoacidosis — a life-threatening complication of diabetes that happens when the body lacks enough insulin, causing it to break down fat for fuel and build up ketones (acids) in the blood.
FSSAI’s stance over the years
Note that this is not the first time the case of ORS has been brought into the public domain.
In 2022, Dr Sivarajani Santosh had filed a PIL with the Telangana High Court seeking the ban of products masquerading as ORS. The same year, on April 8, FSSAI released a statement acknowledging that it had received several complaints and representations regarding the “misuse of the term ORS by certain fruit-based or non-carbonated or Ready-to-Drink beverage manufacturers licensed under FSSAI by labelling/using terms similar to the ORS like ‘ORSL’, ‘ORSL Rehydrate’, ‘Electro Plus ORS’, etc.”
It further said that using the term ‘ORS’ or similar expressions on food product labels or advertisements is not permitted under the Food Safety and Standards Regulations. Such use may classify products as ‘Misbranded Food’ under the FSS Act, 2006, making companies liable for penalties.
While the order did say that labelling such products as ORS or names which could be misinterpreted by consumers as original ORS is a violation of the FSS Act, it did not specifically call for a blanket ban on them.
On July 14, 2022, FSSAI issued another statement reviewing its previous directive, which restricted the use of the term ‘ORS’ on food product labels and advertisements, following representations from food business operators and directions from the Delhi High Court. The regulator noted that some FBOs held valid trademarks for the term ‘ORS’ and sought a review of these trademarks from the Controller General of Patents, Designs and Trademarks. Until then, it allowed such FBOs to continue manufacturing under their registered brand names, provided they display a front-of-pack declaration stating that the product is not a WHO-recommended ORS formula. Those without valid trademarks would have to discontinue the use of the term and submit an undertaking to FSSAI, with six months allowed to clear existing stock.
Then, on February 2, 2024, FSSAI issued a new order allowing food companies with valid trademarks on the term ‘ORS’ to continue using it in their brand names. This followed after clarification CGPDTM, which said that words like “ORS” with a prefix or suffix may be used as a whole under Section 17 of the Trade Marks Act, 1999. The order modified FSSAI’s 2022 directive that restricted such usage. However, it did say that companies would have to clearly state on the front label that the product is not a WHO-recommended ORS formula and also include a disclaimer that the name is only a brand or trademark.
Independent journalism that speaks truth to power and is free of corporate and political control is possible only when people start contributing towards the same. Please consider donating towards this endeavour to fight fake news and misinformation.




